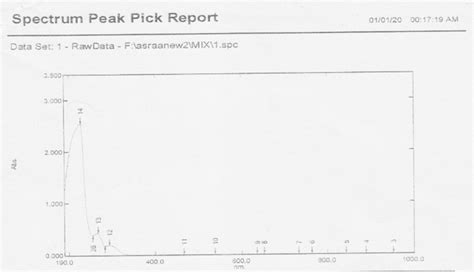
uv vis spectrum analyzer ketamine has a pick uv vis|Pure ketamine extract measurement by UV (Ultra : exporter exporters exporting Materials and methods: Ketamine powder was prepared from precipitation of ketamine hydrochloride solution and characterized by Infrared Spectroscopy (IR), Ultraviolet (UV), Micro elemental. Resultado da AUD-20231218-WA0002
{plog:ftitle_list}
"Crazy" is a song by American hard rock band Aerosmith and written by Steven Tyler, Joe Perry, and Desmond Child. It was the final single from their massively successful 1993 album Get a Grip, released in May 1994 by Geffen Records. "Crazy" peaked at number 17 on the US Billboard Hot 100, number three in Canada, and number one in Iceland for two weeks. In Finland and the United Kingdom, it was released as a double-A side with "Blind Man", reaching number eight in t.
Pure ketamine extract measurement by UV (Ultra
The UV–vis spectrum of chemosensor 1, recorded in a mixture of DMSO/H 2 O 95:5 as solvent (5 × 10 −5 M), shows an intense absorption band at λ max = 507 nm (ε = 69400 cm .Materials and methods: Ketamine powder was prepared from precipitation of ketamine hydrochloride solution and characterized by Infrared Spectroscopy (IR), Ultraviolet (UV), Micro elemental.If the isoprene spectrum on the right was obtained from a dilute hexane solution (c = 4 * 10-5 moles per liter) in a 1 cm sample cuvette, a simple calculation using the above formula indicates a molar absorptivity of 20,000 at the maximum . Notice that the convention in UV-vis spectroscopy is to show the baseline at the bottom of the graph with the peaks pointing up. Wavelength values on the x-axis are generally measured in nanometers (nm) rather than in cm-1 as is the convention in IR spectroscopy. Typically, there are two things that are noted and recorded from a UV-Vis spectrum.
UV–Vis spectroscopy in non-destructive testing. Khalisanni Khalid, . Zaira Zaman Chowdhury, in Non-Destructive Material Characterization Methods, 2024. 15.9 Conclusion. UV–Vis spectroscopy is a cost-effective, simple, versatile, non-destructive, and analytical technique, which is suitable for a large spectrum of organic compounds and some inorganic species.While interaction with infrared light causes molecules to undergo vibrational transitions, the shorter wavelength, higher energy radiation in the UV (200-400 nm) and visible (400-700 nm) range of the electromagnetic spectrum causes many organic molecules to undergo electronic transitions.What this means is that when the energy from UV or visible light is absorbed by a .
sheep colostrum refractometer
Instrument Designs for Molecular UV/Vis Absorption. Filter Photometer. The simplest instrument for molecular UV/Vis absorption is a filter photometer (Figure 10.25), which uses an absorption or interference filter to isolate a band of radiation. The filter is placed between the source and the sample to prevent the sample from decomposing when exposed to higher energy radiation.1 Basic Principles of UV-Vis Measurement 3 1.1 The electromagnetic spectrum 3 1.2 Wavelength and frequency 3 1.3 UV-visible spectra 3 1.4 Transmittance and absorbance 4 1.5 Summary 4 2 How Does a Modern UV-Vis Spectrophotometer Work? 5 2.1 Instrumental design 7 3 Selecting the Optimum Parameters for your 13 UV-Vis Measurements In carefully chosen simple cases (which is all you will get at this level), if you compared the peaks on a given UV-visible absorption spectrum with a list of known peaks, it would be fairly easy to pick out some structural features of an unknown molecule. Lists of known peaks often include molar absorptivity values as well. 2.1 Analysis of Blood Components and Related Species 2.1.1 Bilirubin Analysis. Upstone gives an excellent example of why absorption spectroscopy is still relevant in the face of modern methods for bioanalysis in his review on the use of UV-Vis light absorption spectrophotometry in clinical analysis (Upstone 2013).Patients suffering from a suspected .
UV and visible absorption of transition metal complexes. Ultraviolet and visible absorption spectroscopy involve transitions between electron energy levels in atoms and molecules where the energy difference corresponds to the ultraviolet and visible regions of the electromagnetic spectrum. Wales. A/AS level. WJEC ChemistryThe Thermo Scientific NanoDrop One/One C Spectrophotometer is a compact, stand-alone UV-visible spectrophotometer for measurement of highly concentrated samples across a wide variety of analytes without the need for dilution. A glove-compatible touchscreen offers easy access to preloaded software applications and enables purity and concentration assessments in seconds.
UV-Visible spectroscopy has now been established as an important and fundamental technique in the physical, chemical, biological, engineering, material, and NS laboratory [2,4,112,113].Moreover, UV-Visible spectroscopy has emerged as the most reliable technique to characterize NM optical properties, electronic structures, size, size distribution, state of . UV-Vis can be used to obtain a spectrum of colored compounds. In Figure 1A, the absorbance spectrum of a blue dye is shown. The background shows the colors of light in the visible spectrum. The blue dye has a λ max absorbance in the orange/red. Figure 1B shows a spectrum of a red dye, with λ max in the green. This review highlights in situ UV–vis–NIR range absorption spectroscopy in catalysis. A variety of experimental techniques identifying reaction mechanisms, kinetics, and structural properties are discussed. Stopped flow techniques, use of laser pulses, and use of experimental perturbations are demonstrated for in situ studies of enzymatic, homogeneous, . Eight edible and non-edible oils have been characterized using UV–Vis absorption and transmission, FTIR, and Raman spectroscopy. Oils’ molecular structure, characteristics, and type of bonds have been analyzed separately. Black seed oil and Olive oil have the maximum number of absorbance peaks in the UV–Vis range. There are absorbance .
This compound absorbs light in the UV range due to the presence of conjugated pi-bonding systems. You’ll notice that this UV spectrum has only one peak, although many molecules have more than one. Notice also that the convention in UV-vis spectroscopy is to show the baseline at the bottom of the graph with the peaks pointing up.
The UV-vis region of energy for the electromagnetic spectrum covers 1.5 - 6.2 eV which relates to a wavelength range of 800 - 200 nm. The Beer-Lambert Law, Equation \ref{1} , is the principle behind absorbance .
While interaction with infrared light causes molecules to undergo vibrational transitions, the shorter wavelength, higher energy radiation in the UV (200-400 nm) and visible (400-700 nm) range of the electromagnetic .Shimadzu offers a complete UV-Vis-NIR product lineup, including the versatile single-beam UV-1280 UV-Vis spectrophotometer, the UV-1900i UV-Vis spectrophotometer with an ultra-fast scanning function of 29,000nm/min to enhance your routine analysis, the introductory research-grade UV-2600i/2700i UV-Vis spectrophotometer, the research-grade UV .UV Vis spectrophotometry is used in the pharmaceutical industry to analyze the purity, identity, and strength of drugs. It can also be used to monitor the degradation of drugs over time. To learn more about how spectrophotometry can ensure therapeutic quality, read our customer story.
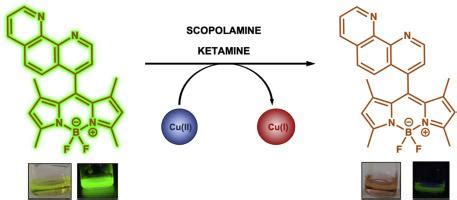
Optical detection of scopolamine and ketamine with a BODIPY
Dye Concentration Using UV-Vis v5 2 • Molar absorptivity – a measure of how strongly a sample absorbs light at a given wavelength; it is a physical property of a compound • Organic molecule – a molecule that contains carbon • Solute – the component in lesser amount in a solution • Solvent – the major component of a solutionUltraviolet–visible (UV–visible) spectrophotometry is primarily a quantitative analytical technique concerned with the absorption of near-UV (180–390 nm) or visible (390–780 nm) radiation by chemical species in solution.These regions of the electromagnetic spectrum (see Table 1) provide energy that gives rise to electronic transitions.The various colors of visible light and .
The UV-vis spectroscopy process is very quick, only taking a few minutes with preparations. UV-vis spectroscopy is extremely accurate and sensitive, which can be crucial in safety-related testing. UV-vis spectrophotometers are relatively inexpensive, compact, and easy to use. They are highly automated and don’t require extensive training.UV-Visible Spectrum The diagram below shows a simple UV-visible absorption spectrum for buta-1,3-diene. Absorbance (on the vertical axis) is just a measure of the amount of light absorbed. One can readily see what wavelengths of light are absorbed (peaks), and what wavelengths of light are transmitted (troughs). The higher the value, the more%PDF-1.6 %âãÏÓ 600 0 obj > endobj 624 0 obj >/Filter/FlateDecode/ID[0D68C31A8E0937438E797621B9C54FBD>81B828330A4A3B4A91845ACEAE23EA1A>]/Index[600 45]/Info 599 0 R .Beckman DU640 UV/Vis spectrophotometer. Ultraviolet (UV) spectroscopy or ultraviolet–visible (UV–VIS) spectrophotometry [1] [2] [3] refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum. [2] Being relatively inexpensive and easily implemented, this methodology is widely .
Depending on the substance, the UV or visible light rays are partially absorbed by the sample. The remaining light, i.e. the transmitted light, is recorded as a function of wavelength by a suitable detector. The detector then produces the sample's unique UV Vis spectrum (also known as the absorption spectrum).It’s easy to enhance the capabilities of your UV-Vis and Vis spectrophotometers with the right accessories and software. Upgrade your instrument hardware with a broad range of accessories for single- and multi-cell sample holders, temperature control units, sipper systems, fiber optic probes, interface cables, replacement lamps, standards, and more.
22 de jan. de 2018 · 0:00 / 3:11. AnaVitória NAMORADAS? | Analisando AnaVitória. Somos todos Harmonizers. 20.1K subscribers. Subscribe. 5.1K. 248K views 5 years ago. .
uv vis spectrum analyzer ketamine has a pick uv vis|Pure ketamine extract measurement by UV (Ultra